PATIENT RESOURCES
An Important Update Regarding Our CAP-002 Program: A Letter to the STXBP1 Community, January 8, 2026
We have provided an update about our ongoing work to understand what happened in the SYNRGY clinical trial, which is now on clinical hold. We have been carefully examining all available information and, while we have not yet identified a root cause, we continue to investigate this unexpected and tragic event. We will share more updates with the medical and patient communities as we have more information to share.
OVERVIEW
At Capsida Biotherapeutics, our unwavering commitment to patients drives everything we do. We are dedicated to finding transformative treatments for serious diseases with significant unmet need. We strive to bring hope and healing to individuals and families affected by devastating conditions. Our cutting-edge research and innovative approach are focused on developing life-changing therapies for those who need them most.
Capsida’s Gene Therapy Approach
Capsida is a clinical-stage, genetic medicines company focused on creating a new class of targeted, gene therapies.
Genes are like instruction manuals for building and operating living things. They contain the information needed to create proteins, which are the building blocks of cells and perform most of the functions in our bodies. Proteins do work in our bodies such as repairing tissue, performing important enzymatic reactions, defending against bacteria, supporting the appropriate transport and localization of molecules and other proteins, and breaking down food during digestion.
Gene therapies aim to deliver the instructions to produce proteins that can potentially overcome diseases caused by genetic mutations that impact specific proteins. For example, in diseases due to a deficiency in a certain protein, gene therapy can deliver genetic information that increases the level of that protein in the cell.
First generation gene therapy approaches for central nervous system (CNS) diseases have typically used naturally occurring viruses to deliver genetic information, but these approaches have had significant limitations. Intravenous administration of these naturally occurring viruses reaches only <1% of neurons in the brain, which is too low to address most CNS diseases. To get around this, many therapies are being delivered by direct administration that involves invasive brain surgery methods, and typically results in only localized delivery of the therapy to certain brain regions, for example those closer to the administration site, rather than allowing for the brain-wide protein expression that is important for maximizing potential therapeutic benefit.
Importantly, Capsida is developing gene therapies that are intravenously (IV)-delivered and use viruses that have been specifically modified to be more effective in crossing the blood brain barrier than naturally occurring viruses. Intravenous delivery uses the body’s natural blood flow to reach more regions of the brain, potentially increasing the treatment’s effectiveness compared to local delivery, and avoids more invasive procedures that are typically required for direct administration to the brain.
Our investigational gene therapy candidates are also detargeted from organs like the liver in animal studies, with the goal of improving safety.
For more information about gene therapies in general, see ASGCT Gene Therapy 101.
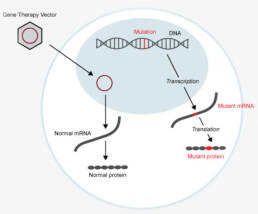
STXBP1 Developmental and Epileptic Encephalopathy (STXBP1-DEE)
STXBP1-DEE is a severe developmental disorder that affects up to 1 in 26,000 newborns globally. This condition is associated with early onset seizures, severe delays in development, intellectual disabilities, and motor disorders. Currently, there are no therapies that can slow down or stop the disease; care focuses on managing symptoms and includes anti-seizure medications and supportive therapies.
Our Approach: CAP-002
CAP-002 is a new kind of investigational gene therapy given through an IV, designed to deliver a working version of the STXBP1 protein to the brain with just one dose. It is designed to avoid organs such as the liver as much as possible and focuses on fixing the root cause, with the goal of changing the course of the disease.
CAP-002 has received FDA Investigational New Drug (IND) clearance. CAP-002 was granted Orphan Drug and Fast Track Designations by the FDA.
The FDA’s Orphan Drug Designation is intended to encourage the development of treatments for rare diseases, defined as those affecting fewer than 200,000 people in the United States. Orphan Drug Designation provides closer FDA collaboration on this program, potential qualification for programs that could expedite development, and certain other benefits. The FDA’s Fast Track designation is designed to facilitate the development and expedite the review of drugs that are intended to treat serious or life-threatening conditions and have the potential to address an unmet medical need.
More information on the Phase 1/2a SYNRGY study is available at clinicaltrials.gov (NCT06983158).
For more information and support, visit the STXBP1 Foundation, a dedicated non-profit organization for patients and families affected by STXBP1.
Meet Reece and the Zink family, who embody incredible strength, resilience, and love. Reece has STXBP1-DEE.
Parkinson’s Disease Associated with GBA Mutations (PD-GBA)
Parkinson’s disease (PD) is a prevalent neurodegenerative disorder, affecting over 10 million adults worldwide. Parkinson’s disease with GBA mutations is a form of Parkinson’s where the disease is linked to changes in a specific gene called GBA1. Up to 15% of PD patients have this form of the disease, making it the most common genetic risk factor. The GBA1 gene tells the body how to make the glucocerebrosidase enzyme. Reduction in levels of this enzyme affects how the body breaks down certain substances, leading to damage in the brain and causing symptoms like tremors, stiffness, slow movement, and cognitive decline. People with this mutation may experience Parkinson’s disease at an earlier age and have faster progressing symptoms compared to those with the other forms of the disease.
Our Approach: CAP-003
CAP-003 is a new kind of investigational gene therapy given through an IV, designed to deliver to the brain a working version of glucocerebrosidase enzyme that is reduced in PD-GBA with just one dose. This therapy was designed to avoid organs such as the liver as much as possible and focuses on fixing the root cause of the condition, with the goal of changing the course of the disease. CAP-003 targets critical brain regions associated with PD-GBA, including the substantia nigra, caudate nucleus, putamen, cortex, and thalamus.
CAP-003 has received FDA IND clearance. More information on the Phase 1/2 study is available at clinicaltrials.gov (NCT07011771).
For more information and support, visit the Parkinson’s Foundation and the Michael J. Fox Foundation for Parkinson’s Research, both of which provide valuable resources for patients and families.
Friedreich’s Ataxia
Friedreich’s Ataxia (FA) is a rare inherited disease that causes a gradual loss of coordination and balance, especially in walking and using the arms and legs. It can also lead to heart problems and loss of sensation in limbs and extremities over time. FA affects about 1 in every 50,000 people, approximately 5,000 patients in the U.S. and 15,000 worldwide.
The condition is caused by a genetic mutation that reduces the amount of a key protein called frataxin. Without enough frataxin, the cells, especially in the brain, spinal cord, and heart, can’t produce energy properly. This leads to stress inside the cells and eventually causes them to die. Since nerve and heart cells can’t easily regenerate, the damage to the nervous and cardiac systems are often the most serious. Currently, there are limited treatment options for FA.
Our Approach: CAP-004
CAP-004 is a new kind of potential investigational gene therapy delivered through an IV, designed to provide a working copy of the frataxin gene to key areas of the body including the brain, spinal cord, heart, and sensory nerves. The goal of this treatment is to give the body a lasting supply of the frataxin protein which is present at low levels in people with FA.
By restoring frataxin levels, CAP-004 has the potential to help cells produce more energy, prevent damage, and slow or stop disease progression. This approach has the potential to address the main effects of FA which include problems with movement, heart function, and sensation, with just one dose. CAP-004 is intended to reach cells that are important for reducing the effects of FA while avoiding organs such as the liver as much as possible. Capsida’s goal is to enable long-term disease modification and substantially slow disease progression, reducing treatment burden.
Visit the Friedreich’s Ataxia Research Alliance for more information and support for patients and families.

